Since its inception in FY 2019, the scale of Utah’s medical cannabis program has increased dramatically. As of September, the Department of Health and Human Services (HHS) reports over 104,000 patients with active medical cannabis cards. Administering and implementing the medical cannabis program is a unique partnership between HHS and the Department of Agriculture and Food (UDAF). Unlike many of Utah’s state programs, medical cannabis is entirely self-funded (a statutory requirement). This post serves as an overview of the program’s structure, provides an update on revenues, and summarizes recent policy changes.
Patient Focus
HHS houses the Center for Medical Cannabis, which licenses medical cannabis patients, providers, and also provides staff support to the Medical Cannabis Policy Advisory Board.
HHS is authorized to charge fees for licensure of patients and providers. Prior to July 1, 2025, that included Qualified Medical Providers who recommend medical cannabis to patients. Those providers will no longer pay a fee to HHS, and will be overseen by the Division of Professional Licensing. Another substantial change from the 2025 General Session was reducing the Uniform Transaction Fee by 50%. This fee is applied to all medical cannabis purchases (over 1.6 million transactions in FY 2025 alone). The table below shows the categories of fees charged by HHS along with the amount of revenue collected in FY 2025.
| HHS Fee Name | Fee Amount | Revenue |
|---|---|---|
| Guardian and Provisional Card Fees | $15 – 68.25 | $2,789 |
| Uniform Transaction Fee | $3.00 | $4,827,825 |
| Caregiver Card Fees | $5 – 68.25 | $21,748 |
| Patient Card Fees | $15 | $1,688,611 |
| Pharmacy Medical Provider Fees | $50 – 150 | $5,400 |
| Qualified Medical Provider Fees | $50 – 150 | $42,340 |
| Interest Income | – | $421,373 |
| Total FY 2025 Revenue | $7,010,085 |
If the 2025 General Session policy changes had applied to the medical cannabis industry in the last fiscal year, patients and providers would have saved over $2.5 million in fees. The chart below shows the activity of the Qualified Patient Enterprise Fund (Patient Fund), which represents the entire budget for the HHS medical cannabis program.
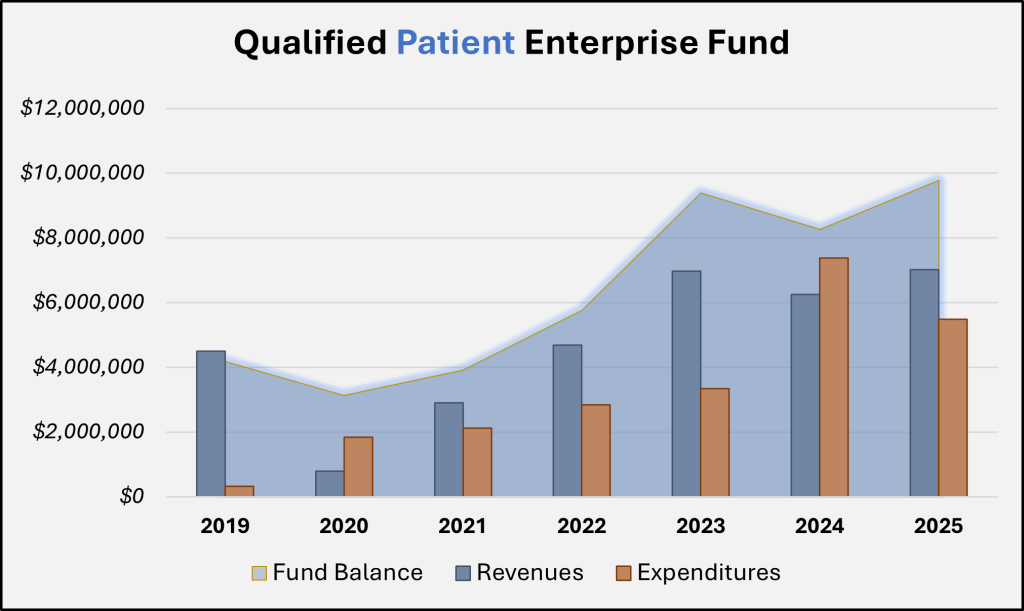
Enterprise Funds are used to account for government operations that function like a private business. These funds do not typically require appropriation, and are designed to recover costs primarily through user charges (fees for services and regulatory oversight). As can be detected in the graph for the Patient Fund, the account’s balance has grown steadily since 2019. When the program was created, the Legislature allocated $4.5 million one-time General Fund to the (former) Department of Health and $900,000 to UDAF as seed money for the two arms of the program. As of FY 2024, both of these ‘loans’ have been repaid by each program. The preliminary yearend balance for the Patient Fund is nearly $9.8 million.
The chart below describes the number of patients who registered or renewed their cards by month in the state, along with the number of pharmacy transactions statewide.
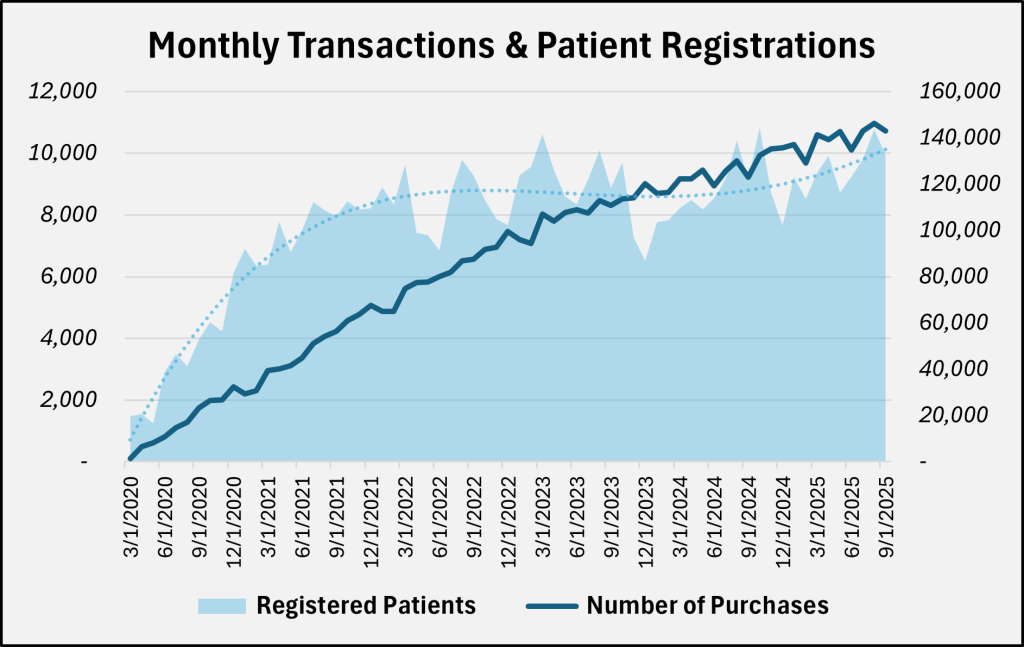
In addition to reducing the uniform transaction fee, HHS reduced the fees paid for patient registration by Utah residents. The graph above shows since the program’s beginning the number of transactions has steadily increased each month. While the growth in monthly patient registrations has somewhat stabilized, the total number of patients continues to increase, adding nearly 15,000 patients from FY 2024 to FY 2025. That is to say, even with the significant reductions in the FY 2026 fee schedule, the HHS Medical Cannabis program is expected to be well funded into the foreseeable future.
Production Focus
UDAF’s medical cannabis responsibilities include regulating cultivators, processors, and pharmacies, as well as staffing the Cannabis Production Establishment and Pharmacy Licensing Advisory Board. Until the 2023 General Session, pharmacy oversight was conducted by HHS.
UDAF’s fee structure differs from HHS in that several of the entities they are allowed to charge fees to are capped in statute. There are currently eight licensed cultivators in the state. Per UCA 4-41a-205, UDAF can’t issue additional cannabis cultivation facility licenses unless an existing license is forfeited, current cultivators fully utilize their 100,000 square foot production limit, or if patient demand (as determined by HHS) overwhelms the current supply.
| UDAF Fee Name | Fee Amount | Revenue |
|---|---|---|
| Establishment Application Fees | $250 – $2,500 | $7,300 |
| Cultivator License | $100,000 | $800,000 |
| Processor License Fees | $35,000 – $90,000 | $1,385,000 |
| Pharmacy Licenses | $33,500 – $67,000 | $887,000 |
| Courier/Home Delivery Licenses | $2,500 | $17,500 |
| Laboratory License | $15,000 | $15,000 |
| Establishment Agent Cards | $100-$150 | $147,665 |
| Research University License | $2,500 | $0 |
| UDAF Analytical Laboratory Testing | Varies by test | $253,180 |
| Other (grama, reprints, etc.) | – | $3,685 |
| Citation Revenue (transferred to General Fund) | – | $44,100 |
| Interest Income | – | $128,458 |
| Total FY 2025 Revenue | $3,644,788 |
This same section of code limits the number of Tier 1 processor licenses to 18, though last year UDAF only issued 15. Tier 1 Processing Licenses allow entities to process, formulate, package, and label products, where as a Tier 2 license only allows packaging and labeling.
Similarly, UCA 4-41a-1005 allows up to 17 Pharmacy licenses; this threshold was increased by two licenses during the 2025 General Session. H.B. 54, “Cannabinoid Amendments” enacted UCA 4-41a-1006 which outlines the timeline and eligibility for awarding the two additional pharmacy licenses, including that they can’t be financially related to an existing pharmacy and must be located in rural counties that are designated as medically underserved by the U.S. Department of Health and Human Services.
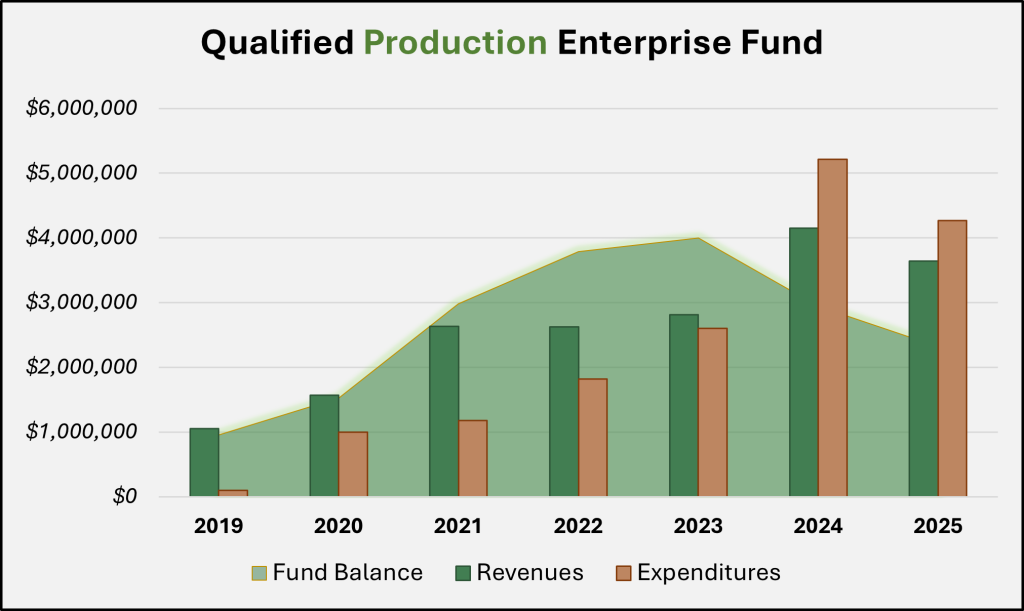
Based on this statutory criteria surrounding licensure, it’s perhaps not surprising that the fee revenue for the UDAF program was about half that of the HHS program in FY 2025. The Qualified Production Enterprise Fund (Production Fund) closed FY 2025 with a balance of $2.3 million, or roughly a quarter of the Patient Fund.
Legislative Oversight
In the 2022 General Session, the Legislature passed S.B. 153, “Medical Cannabis Governance Study” which created the Medical Cannabis Governance Structure Working Group. This group, comprised of legislators from both the Health and Human Services Interim Committee and the Natural Resources, Agriculture, and Environment Interim Committee, was initially tasked with determining if oversight of the program should be housed under a single state agency. The following year the membership was broadened to six members of the legislature and the committee’s scope of work was extended to making recommendations to both interim committees about the governance of the Utah medical cannabis program.
In the 2025 General Session, the Legislature passed the following bills related to medical cannabis (which were almost entirely sponsored by members of the governance structure working group):
- S.B.64, “Medical Cannabis Amendments”: set limits for Tier 1 processing facilities and requires HHS to create patient product information inserts to include dosing information, instructions, side effects, safe storage recommendations, etc.
- H.B. 54, “Cannabinoid Amendments”: allows for two additional pharmacy licenses, reduces fees for certain rural pharmacy licenses, requires UDAF to report on fines issued to regulated entities, and allows processors to include certain product information online.
- H.B. 343, “Cannabis Production Amendments”: requires cultivators, processors and labs to include odor control in their operating plans and requires UDAF to make recommendations to the governance structure working group about standards for odor control.
- H.B. 357, “Medical Cannabis Modifications”: eliminates qualified medical providers, sets standards for continuing education required for recommending medical providers, and shifts regulation for medical providers to the Department of Commerce.
Through it’s first seven years in practice, Utah’s medical cannabis program has seen robust adoption by patients which has offered opportunities for production establishments, pharmacies, and regulators to develop a program that is both unique to Utah and responsive to larger industry standards. Active policy refinement and budgetary oversight by the legislature is paving the way for an affordable, self-funded program that serves constituents in even the most rural areas of the state.